- What is happening to the outside air pressure today?
- If we wet the inside of a bell jar, add some smoke, and then pump the air out of the jar using a vacuum pump, will a cloud form inside the jar?
- A "front" is where two different air masses meet. Cold fronts and warm fronts have different shapes, which cause them to produce different types of weather events. Both bring precipitation, but one brings more intense precipitation. Look at the picture on the board... Which front will produce more intense precipitation? Why are they shaped differently -- isn't warm air hitting cold air the same thing as cold air hitting warm air????
Today:
- Vacuum Pump Demo
- Block 2: Finish Handout 2: Practice Drawing Climate Maps
- Get test review
Homework:
- Block 1: Complete Weather/Climate Questions 1-14.
- Block 2: Complete North America Climate Map and Practice Map A.
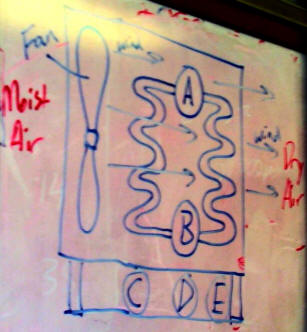
I. The diagram on the right shows a simple dehumidifier.
- If A is the position of a compressor, and B is the position of an expansion valve, which way should the fluid flow? Clockwise or counter-clockwise?
- Where should the drip pan be located? At C, D, or E? Why?
- Why is it a problem for the refrigerant to flow in the opposite direction?
II.
- As air rises, which type of air cools off faster, dry air (low relative humidity) or wet air (100% relative humidity)?
- All other things being equal, which air is more dense, air with low or high relative humidity?
- Why?
III. The How can you get a boiled egg to fit through the neck of a narrow mouthed glass flask?
Today:
- Continue Handout 2: Practice Drawing Climate Maps
Homework:
Block 1:
- Complete climate maps of practice continents A and B.
- Quiz next class: be able to draw climate maps on continents such as A and B.
Block 2: None
"This I Believe" essay by Jon Carroll
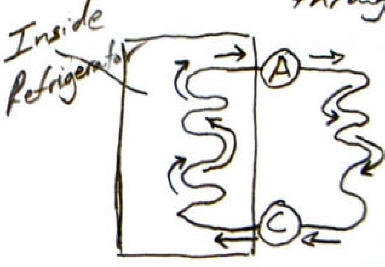
The diagram on the right shows a simple refrigerator. Arrows indicated the direction of "refrigerant" flow through a tube. The positions of a compressor and an expansion valve are indicated as circles.
- At which letter is the expansion valve located?
- At which letter is the compressor located?
- Why does the refrigerant meander?
- What makes a good refrigerant?
Today:
- Return tests
- Block 1: continue climate map drawing
- Block 2: finish Handout 1: Cloud Formation and begin Handout 2: Practice Drawing Climate Maps
Homework:
1. How do home canning jars work?


2. The plume of "smoke" below is mostly water. Is that water probably a solid, liquid, or a gas?

Today:
- Correction to #5 solution on test review part II.
- Test next class. Mr. Stapleton will be at preschool. Last chance to ask about test items.
- If there's time, finish Handout 1: Cloud Formation and begin Handout 2: Practice Drawing Climate Maps
Homework:
- Study
- If you have colored pencils, bring them to class on Monday.
- Why does human skin tend to become drier in the winter?
- Why are basements more damp in the summer?
- What is climate?
- How can you create over 100 smoke rings by using six playing cards and by blowing out just one birthday candle?
Today:
- #16 grading
- Finish going over Test Review Part I.
- Questions about Test Review Part II: Problems ?????
- Begin short unit: using physics to understand weather and deduce climate patterns. (Handout 1: Cloud Formation) (Handout 2: Practice Drawing Climate Maps)
Homework:
- If you have colored pencils, bring them to next class.
- Study -- go through test review part II.
It is often the case that a cork floating in a narrow glass jar will "want" to move to the side of the jar. Without touching the cork, how can you make it stay in the middle of the jar? [There are at least two simple solutions.]
Today:
- Check and discuss pressure review part I. Below is a video of 2nd block discussion of #1-10 and the last one. In my discussion of the helmet question, I started into an example that I'm afraid might have left some of you more confused than you were already. Here's a simple way to look at the helmet question... Imagine that your head is made of snow. If you have to allow tiny people to walk around on it, would you prefer that they wear boots or snowshoes? The smart answer is snowshoes, because snowshoes will decrease the pressure applied by your pedestrians. The pedestrians' force remains the same, but it is spread over a larger area. Likewise, a helmet -- even a hard one -- spreads out the force of impact and lessens the possibility of skull penetration.
- Questions about the problems???
- Begin short unit: using physics to understand weather and deduce climate patterns.
Homework:
- Test next Thursday. Questions will be selected from the test review questions and problems.
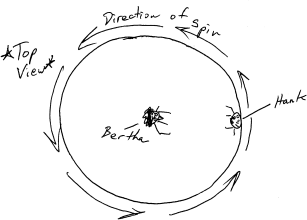
Hank and Bertha are sitting on a merry-go-round that is rotating counter-clockwise. They begin throwing things at one-another while the merry-go-round continues to turn.
The Coriolis Effect can cause thrown objects to appear to curve as they leave the thrower. As Hank and Bertha throw their projectiles, who sees her/his projectiles curve to the right? Who sees his/her projectiles curve to the left? Who sees no curve?
Today:
- Check for homework Blank template for #16
- Hovercraft Stuff: Create a graph of coefficient of friction vs. payload. Attempt a perfect hovercraft stop. Coriolis simulation -- hopefully.
Homework:
- *Complete part 1 of Pressure Test Review by next class.
- *Read through/practice part II of Pressure Test review (interactive problem set).
- Tentative test day -- next Tuesday (3/22)
- We used an "air source" to inflate hot air balloons and thereby measure their volumes. How can we determine the gauge pressure produced by that air source?
- Can we use the air source and a heavy duty trash bag to lift a student? Why or why not?
- Can you think of three different equations (with none of the same variables) for pressure? [Think of a third one may be tricky.]
Today:
- Questions about the pressure problems?
- Explanation of homework -- particularly 16
- Hovercraft Questions and experimentation
- Work time -- homework, excel
Homework: Due on Monday
- Complete "more pressure problems" 13, 14, and 16-18. You can skip 15.
- How does siphoning work?
- Can you siphon without gravity? What about without an atmosphere?
- What's a water level? How does it work?
Today:
- Problems 8b-12.
- ????
- Mini-hovercrafts -- modify for maximum distance.
Homework:
- Block 1: Watch video of solutions to homework problems.
- Due next Thursday -- Problem #16 -- graph of liftable mass vs spherical balloon radius.
A lot of igloos end up looking more like castles; their walls are too vertical to form a dome. Can you think of a "fool-proof" way to build an actual dome? Link to igloo project video



Today:
- Announcement
- BHS Arts Festival May 27th
- Check for homework problems that were due on 2/11. Review Problems. Here are the solutions to ALL of the problems, including the missing parts of 8a and 8b. See below for video solution. You will need to view this on full screen. And if you have an HD monitor, make sure you turn it on in the video.
- Work time -- Balloon calculations
- Complete balloon calculations. Enter solutions into Excel spreadsheet so that Mr. Stapleton can easily check your answers. Fill in all of the blanks. Due Friday.
Homework:
- Balloon calculations
Other:
Link to sledding video with soundtrack.
What does the Gateway Arch have in common with a really, really, strong igloo -- but not a bad ski jumper?

Today:
- Get balloons flying. Collect data -- either mass lifted or reduction of felt mass.
- Enter your data into this Excel spreadsheet. Fill in all of the blanks.
Homework:
Complete balloon calculations. Enter into Excel Spreadsheet, above, and turn in by Friday after break (March 4th)
What do a good igloo and a bad ski jumper have in common?
Today:
Homework:
See last class
- What's the opposite of a suction cup?
- What's the principle that makes snowshoes work?
- Why are longer skis generally faster?
Today:
- Ho much pressure can you generate?
- Work time: problems and/or balloons
- Balloons must be "flown" by the end of class next Thursday.
- Next class -- something in the snow.
Homework:
1. Bring snow attire next class.
2. Balloons must be "flown" by the end of class next Thursday. For an A+ on this project, you must use buoyancy to correctly calculate the air temperature inside your bag. These calculations must be done individually, but you may get some help from your friends. If your balloon actually flies on its own, you get an extra 2% added to your grade. If groups would like to fuse their balloons together, that is okay.
2. Problems 1-6, 8-12. Here are my solutions. I haven't double checked them, so there may be some mistakes.

- The molar mass of dry ice is about 44g/mol. If a chunk of dry ice is placed in a 2-liter bottle, and then the bottle is capped, the bottle can explode as the dry ice sublimes (solid to vapor). At 70 degrees Fahrenheit (294.3K), approximately how much dry ice would have to sublime in order for the bottle to explode? Assume that a two-liter bottle can withstand exactly 180psi of internal pressure. Consider the volume of the solid dry ice to be negligible.
- Is this estimate probably a little low or a little high? Explain.
- What are some of the dangers of doing this?
Today:
- Review homework problems. Here's the solution to the boat problem (#14). Work on hot air balloons.
Homework: Generate at least two proposals regarding how we can incorporate the snow into this class. E-mail them to jstaplet@bsdvt.org.
- I have two balloons separated by a closed tube. One is almost fully inflated. One is just partially inflated. What will happen when air is allowed to flow through the tube?
- What's the easiest way you can think of to accurately measure the air pressure inside a balloon?
Today:
- Review homework. See video below. The other one should be posted by tomorrow.
Homework:
Homework Problems 12-14. Time-saving, corner-cutting change to #14**** -- Assume that the steel sheets from which the boat is made have a mass/area ratio of 234kg/m^2, and also assume that they have negligible thickness.
- In pounds, how much heavier would you be if this room were a vacuum?
- Fred put a flat, empty Ziploc bag on a super sensitive balance and recorded the bag's mass. Fred then opened the bag, filled it half-way with room air, sealed it, and placed it back on the balance. When Fred checked the new mass reading, did he see an increase, a decrease, or no change?
Today:
Homework:
- A 40ml rock has a density of 7g/ml. The rock is placed inside a 1 liter Ziploc plastic bag having an empty mass of 10g. The rest of the bag is filled completely with air having a density of 1.2kg/m^3 and a pressure equal to the outside air pressure (101,350pa). The bag is sealed in this state. The bag is then attached to the outside of a submarine and carried downward until the bag begins to sink on its own. If the bag remains intact and loses none of its contents, at what water depth does it begin to sink on its own? Assume that the volume of the plastic in the bag is negligible and that there is no significant temperature change with water depth.
- You place an empty balloon on a balance, and the balloon's mass is 15g. Then you inflate the balloon with dry air and measure the balloon's new total mass. The new mass is 16g. You know that the balloon's volume is 5L, and you know that the current density of the room air is 1.2kg/m^3. If the air pressure is 101,350pa, and there is no temperature change, what is the gauge pressure of the air inside the balloon, in psi? ["gauge pressure" is pressure minus atmospheric pressure].
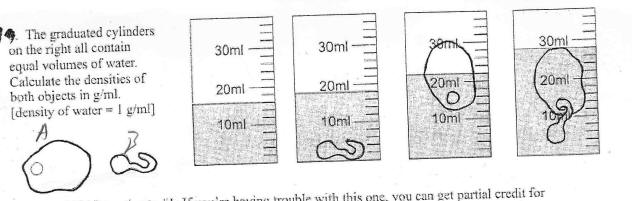
Each of the
graduated cylinders contains the same amount of water. Find the
volumes, masses, and densities of objects A and B.
Today:
Homework: None
 Warm-up:
You would have already tried this, had your bathtub and tub toys been
big enough...
Warm-up:
You would have already tried this, had your bathtub and tub toys been
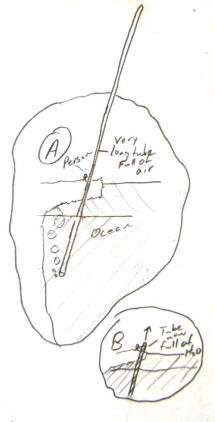
big enough...The pictures on the right show a person holding a very long glass test tube. In picture A, the tube is essentially full of air, though it is leaking a few bubbles from the bottom (because the picture looks more fun that way). The tube is sealed on its top end.
In picture B, the person has somehow filled the test tube with water and submerged it. The tube is being raised from the water, closed end first.
Regarding picture B, if you assume that the tube is 500m long, what will happen to the water level inside the tube as the tube is held vertically and its closed end is raised 499m above the water's surface?
If 1inHg = 3386pa, what's the density of mercury?
Today:
- Attempted Fire Demo
- Scuba Demo
- Hot Air Balloon Practice problem... Find the Fahrenheit temperature inside this hot air balloon... The balloon's volume is 0.4m^3. The total mass of the empty balloon is 0.058kg. At its maximum buoyancy, the balloon has lifted itself, plus 30cm of rope. Each cm of this rope has a mass of 0.0015kg. Current air pressure is 29.92inHg. 1inHg = 3386pa. Assume that the molar mass of air is 28.97g/mol. Givens are in yellow. Steps are black. Solution is in pink.
Homework:
- Complete pressure questions and problems 1-7, 11
- For more help, read p.275-286 in the textbook.
- Explain (in detail) why the blobs in a lava lamp rise and sink.
- I have a film canister with a hole in the lid, some Alka Seltzer, some pennies, and a vessel full of water. Explain how to make the canister sink to the bottom, sit there for a while, and then rise back to the surface on its own?
Today:
- Return Midterms
- Block 2: Finish notes [Link to completed notes. Link to blank notes]
- Problem: density of plumber's putty. Find the density of plumbers' putty using water, liquid measurement containers, and plumbers' putty.
Homework:
- Collect materials for hot air balloon -- due next class.
- pressure questions and problems 1-7, 9, 11-13